Endoscopic Ultrasound Probe
Now you can ultrasound anywhere
EndoSound is a groundbreaking endoscopic ultrasound (EUS) system that integrates real-time ultrasound directly into video endoscopy, pushing equine diagnostics beyond what has ever been possible. By allowing veterinarians to visualize and assess structures beneath the mucosal surface—such as cardiac anatomy, reproductive organs, and deep gastrointestinal tissue—EndoSound opens the door to entirely new diagnostic and research pathways in large-animal medicine.

Downloadable Resources
A Revolutionary Breakthrough
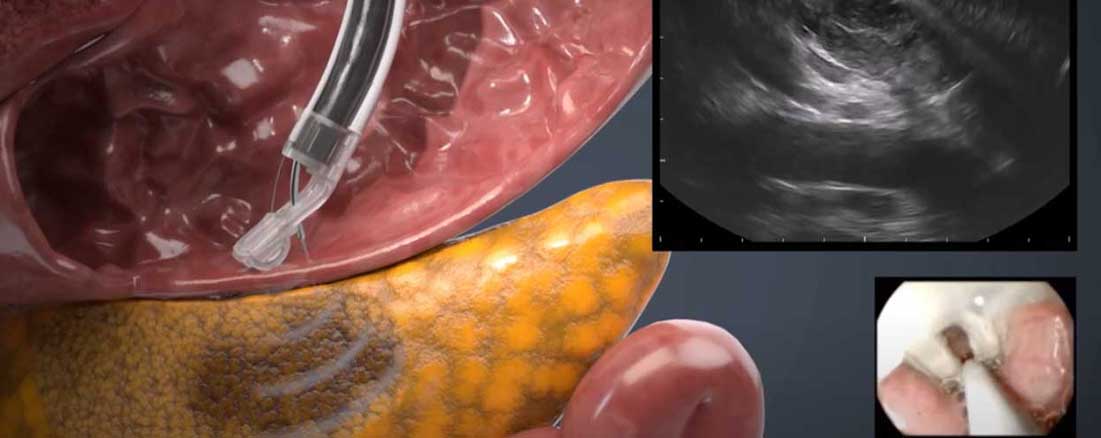
Highly Accessible Ultrasound
1982
First Commercially Available Ultrasound Endoscope
Built by Olympus, offering a radial probe tipped fiberscope
1988-1990
First Linear Ultrasound Endoscope
Challenges With Mechanical Probes Led To The Development by Pentax/Hitachi
1992
FNA Becomes Protocol
Early results showed promise in the detection of cancers in anatomy previously unreachable by ultrasound. Primarily in the pancreas and interstitial lung tissues.
1995
EUS Becomes Gold Standard
The detection results in pancreatic cancer are so positive that the ASGE and the FACG move to make EUS the gold standard in pancreatic cancer detection.
1998-2010
Application Expands
From imaging to intervention, endoscopic ultrasound now enables minimally invasive therapies—transluminal drainage, precision injections, and liver interventions—redefining what can be treated without surgery
2000-2015
Technology Pushes Leaps In Capability
The integration of color Doppler, contrast imaging, and shear wave elastography transformed ultrasound from visualization alone into quantitative tissue intelligence—elevating diagnostic and therapeutic decision-making.
2020
Development Begins On EndoSound Concept
The company’s core technology—now known as the EndoSound Vision System (EVS)—is designed to convert standard flexible upper GI endoscopes into fully functional EUS platforms through a patented attachment mechanism. This approach was developed to expand access to EUS, a critical imaging modality traditionally limited to high-cost, hospital-based systems.
2021
FDA Gives First Nod To EndoSound Concept
By issuing an official “FDA Breakthrough Device” designation. Allowing for expanded clinical testing and trials.
2024
FDA Gives Full Clearance to EndoSound
The full 510(k) approval was granted and full clinical market deployment began. Offering world-class endoscopic ultrasound at a fraction of the market price.
2025
EndoSound Breaks The Species Barrier
In Late 2025, EndoSound and Rutledge Medical began discussions about the widespread application of endoscopic ultrasound in veterinary medicine.
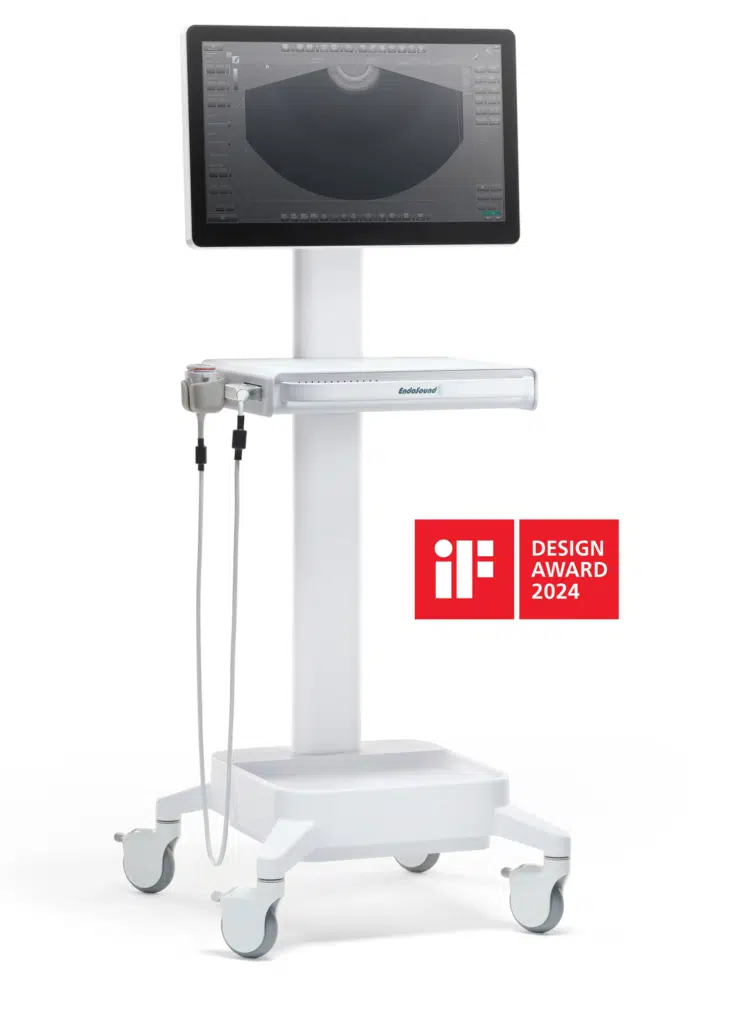
A Simple Addition
Specifications